Fluorescence Microscopes
MicroTime 200 STED
Time-resolved Confocal Fluorescence Microscope with Super-resolution Capability
- Complete confocal STED system based on inverted microscope body
- Optical resolution below 50 nm
- Excitation at 640 nm and optionally with additional lasers at 595 nm and 660 nm
- Up to 6 truly parallel detection channels using SPADs or Hybrid-PMTs
- Supports gated STED (gSTED) and gSTED-FCS
- Piezo scanning for 2D- and 3D-(lifetime) imaging and accurate point positioning
- Advanced easy-to-use data acquisition and analysis software SymPhoTime 64
- Upgrade options for simultaneous AFM/FLIM/STED measurements
- Supports all other methods available for the MicroTime 200, i.e., FLIM, FCS, FCCS, FLCS, FRET, etc.
- FLIMbee galvo scanner add-on with outstanding flexibility in scanning speed and excellent spatial precision
- scanning FCS available via line scan in x using FLIMbee galvo scanner
- Description
- Specifications
- Add-on options
- Videos
- Examples
- Applications
- Images
- Documents
- Publications
In recent years, super-resolution microscopy has gained more and more attention. It has now evolved beyond the stage of development and permits to investigate biological systems that were formerly obscured by the diffraction limit of light. One of the most popular techniques for super-resolution imaging is Stimulated Emission Depletion (STED) microscopy. STED is usually performed with confocal microscopes and is therefore ideally suited to be added to the MicroTime 200. The integration of STED into the system has been driven towards highest robustness and ease-of-use. The system permits to perform STED microscopy without lengthy alignment preparations while still having the choice to modify the system and use the full capability of the open microscopy platform MicroTime 200.
Less than 50 nm optical resolution
 STED microscopy uses the principle of stimulated emission depletion. After exciting fluorophores in the laser focus, a second, donut-shaped focus of a laser with longer wavelength is used to actively de-excite the molecules in the periphery via stimulated emission. In the MicroTime 200 STED, the donut is created using a so-called EASYDOnut phase plate. It is inserted into the beam path and changes the STED laser focus to a donut-shape, while leaving the excitation laser unaffected. This simple implementation makes spatial alignment of the two laser beams, which emerge from the same optical fiber, unnecessary and yields a spatial resolution of less than 50 nm. Due to the time-resolved data acquisition principle of the MicroTime 200 STED, it is additionally possible to apply time-gates to all measured data. This gated STED (or gSTED) approach leads to an enhanced resolution in images and a reduced observation volume for FCS.
STED microscopy uses the principle of stimulated emission depletion. After exciting fluorophores in the laser focus, a second, donut-shaped focus of a laser with longer wavelength is used to actively de-excite the molecules in the periphery via stimulated emission. In the MicroTime 200 STED, the donut is created using a so-called EASYDOnut phase plate. It is inserted into the beam path and changes the STED laser focus to a donut-shape, while leaving the excitation laser unaffected. This simple implementation makes spatial alignment of the two laser beams, which emerge from the same optical fiber, unnecessary and yields a spatial resolution of less than 50 nm. Due to the time-resolved data acquisition principle of the MicroTime 200 STED, it is additionally possible to apply time-gates to all measured data. This gated STED (or gSTED) approach leads to an enhanced resolution in images and a reduced observation volume for FCS.
Choice of excitation wavelengths
 The excitation system of the MicroTime 200 STED is build around a dedicated laser combining unit that integrates the excitation lasers for STED, and the STED laser itself. The standard excitation wavelength is 640 nm, which can optionally be combined with additional lasers at 595 nm and 660 nm for dual species STED imaging. Due to the high power of the STED laser at 765 nm, special care was taken to ensure the user's safety when operating the instrument. A second laser combining unit with additional pulsed diode lasers can also be attached to the system for non-STED applications such as FLIM, FRET or FCS.
The excitation system of the MicroTime 200 STED is build around a dedicated laser combining unit that integrates the excitation lasers for STED, and the STED laser itself. The standard excitation wavelength is 640 nm, which can optionally be combined with additional lasers at 595 nm and 660 nm for dual species STED imaging. Due to the high power of the STED laser at 765 nm, special care was taken to ensure the user's safety when operating the instrument. A second laser combining unit with additional pulsed diode lasers can also be attached to the system for non-STED applications such as FLIM, FRET or FCS.
The laser power and repetition rate can be flexibly adjusted by the multichannel laser driver PDL 828 “Sepia II”, which even allows to address several lasers in parallel enabling advanced techniques like Pulsed Interleaved Excitation (PIE). The time delay between the excitation pulse and the STED pulse is adjustable for highest flexibility and best STED resolution.
Scanning Technologies
 The great versatility of the MicroTime 200 platform is complemented by the FLIMbee galvo scanner which can provide scanning speeds ranging from very slow to fast while maintaining high precision. This high degree of flexibility in speed allows for applications ranging from Phosphorescence Lifetime Imaging (PLIM) to fast fluorescence lifetime measurements using rapidFLIM. Furthermore, with its high precision and sensitivity, the FLIMbee scanner is optimally suited for super-resolution microscopy via STED, enabling imaging down to the single molecule level.
The great versatility of the MicroTime 200 platform is complemented by the FLIMbee galvo scanner which can provide scanning speeds ranging from very slow to fast while maintaining high precision. This high degree of flexibility in speed allows for applications ranging from Phosphorescence Lifetime Imaging (PLIM) to fast fluorescence lifetime measurements using rapidFLIM. Furthermore, with its high precision and sensitivity, the FLIMbee scanner is optimally suited for super-resolution microscopy via STED, enabling imaging down to the single molecule level.
A MicroTime 200 equipped with a FLIMbee scanner is a good choice for Single Molecule Detection (SMD) methods such as spFRET, PIE-FRET, (STED-)FCS, FLCS, FLCCS, dual-focus FCS (2fFCS), and even anisotropy measurements. Additionally, Two-Photon Excitation (TPE) with descanned and non-descanned detection is possible.
The core of the FLIMbee galvo scanner consists of three high precision oscillating mirrors with excellent linearity, repeatability and low drift. The two y-axis galvo mirrors ensure that the laser beam is stationary at the entrance of the objective. This mirror configuration minimizes vignetting of the image field and ensures a constant focal volume over a wide scan range. The FLIMbee scanner provides a minimal pixel size of 10 nm when using a 100x objective.
Use of the standard piezo scanner is recommended for applications requiring light from the UV (255 to 400 nm) and NIR (1100 to 1400 nm) spectral regions or when pixel sizes smaller than 10 nm are desired.
Detection subsystem with single photon sensitivity
 In the MicroTime 200 STED, scanning is facilitated through a piezo table optionally combined with a high precision PiFoc element for 3D imaging. The choice of piezo scanning ensures a high repositioning accuracy and stability, which is essential for high quality STED images. The system can be configured for up to six individual detection channels. For the deep-red emission of the STED dyes depleted at 765 nm, SPADs are typically used, since they feature a high detection efficiency of up to 70% in this spectral range.
In the MicroTime 200 STED, scanning is facilitated through a piezo table optionally combined with a high precision PiFoc element for 3D imaging. The choice of piezo scanning ensures a high repositioning accuracy and stability, which is essential for high quality STED images. The system can be configured for up to six individual detection channels. For the deep-red emission of the STED dyes depleted at 765 nm, SPADs are typically used, since they feature a high detection efficiency of up to 70% in this spectral range.
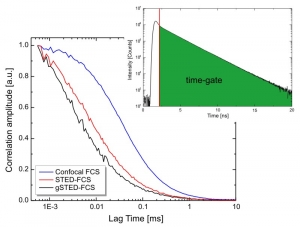
Gated STED with picosecond resolution
 Data acquisition is performed using time-correlated single photon counting (TCSPC) using PicoQuant's unique time-tagged time-resolved (TTTR) data acquisition mode with picosecond resolution. The acquired TTTR data can not only be used for regular STED imaging, but also to perform gated STED (gSTED) measurements. The advantage of TTTR data acquisition mode is that it allows to perform vastly different measurement procedures besides STED, such as FLIM, FCS, or even coincidence correlation (“antibunching”), based on just one fundamental data format. The TTTR format is supported by all available TCSPC electronics from PicoQuant.
Data acquisition is performed using time-correlated single photon counting (TCSPC) using PicoQuant's unique time-tagged time-resolved (TTTR) data acquisition mode with picosecond resolution. The acquired TTTR data can not only be used for regular STED imaging, but also to perform gated STED (gSTED) measurements. The advantage of TTTR data acquisition mode is that it allows to perform vastly different measurement procedures besides STED, such as FLIM, FCS, or even coincidence correlation (“antibunching”), based on just one fundamental data format. The TTTR format is supported by all available TCSPC electronics from PicoQuant.
Intuitive data acquisition and analysis
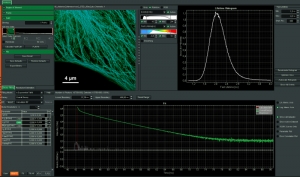 SymPhoTime 64, the MicroTime 200 STED system software, features dedicated STED data acquisition and analysis protocols. For example, preset time-gating is integrated in the measurement preview, and flexible time gates for higher STED resolution can be set during data analysis. The unique fluorescence pattern matching approach can be used to separate multiple species from data recorded with the same STED laser wavelength.
SymPhoTime 64, the MicroTime 200 STED system software, features dedicated STED data acquisition and analysis protocols. For example, preset time-gating is integrated in the measurement preview, and flexible time gates for higher STED resolution can be set during data analysis. The unique fluorescence pattern matching approach can be used to separate multiple species from data recorded with the same STED laser wavelength.
FLIM, FRET, FCS, and more
The MicroTime 200 STED can not only be used to perform STED and STED-FCS, but also supports all other measurement and analysis procedures available for the MicroTime 200. This includes FLIM, FCS, FCCS, FLCS, FRET, PIE-FRET, or intensity time-traces, to name just a few.
| Optical resolution |
|
| Excitation system |
For STED
|
| Microscope |
|
| Objectives |
|
| Scanning |
Piezo:
Galvo scanner FLIMbee:
|
| Main optical unit |
|
| Detectors |
|
| Data acquisition |
|
| Software |
|
Not all options can be combined with each other.
* deep-UV not yet in combination with STED
All Information given here is reliable to our best knowledge. However, no responsibility is assumed for possible inaccuracies or omissions. Specifications and external appearances are subject to change without notice.
Combination with optical tweezers
 As a joint development, PicoQuant and Ionovation combine time-resolved microscopy and optical tweezers in a single system. Both instruments retain their individual functionalities and can also be used together, thus enabling customers to carry out time-resolved fluorescence microscopy (e.g. fluorescence lifetime imaging) on trapped objects such as cells.
As a joint development, PicoQuant and Ionovation combine time-resolved microscopy and optical tweezers in a single system. Both instruments retain their individual functionalities and can also be used together, thus enabling customers to carry out time-resolved fluorescence microscopy (e.g. fluorescence lifetime imaging) on trapped objects such as cells.
Ionovation’s PicoTweezers system is a compact, robust trapping and force measurement device based on the “optical tweezers” concept which is equipped with real-time video detection. In contrast to common optical tweezers systems, PicoTweezers has no spatial restrictions and is compatible with all sample carriers. The system is well suited for
molecular interaction studies, force spectroscopy, cell trapping, rheology and many
other applications.
Combination with AFMs
 The combination of atomic force microscopy (AFM) with single-molecule-sensitive confocal fluorescence microscopy enables a fascinating investigation into the structure, dynamics and interactions of single molecules or their assemblies. AFM reveals the structure of macromolecular complexes with nanometer resolution, while fluorescence can facilitate the identification of their constituent parts. In addition, nanophotonic effects, such as fluorescence quenching or enhancement due to the AFM tip, can be used to increase the optical resolution beyond the diffraction limit. For the first time, this development grants access to the effects of precise physical impact on biomaterials like cells and its effects simultaneously measured by fluorescence parameters. The combination of the MicroTime 200 STED with an AFM has currently been realized for three different AFMs:
The combination of atomic force microscopy (AFM) with single-molecule-sensitive confocal fluorescence microscopy enables a fascinating investigation into the structure, dynamics and interactions of single molecules or their assemblies. AFM reveals the structure of macromolecular complexes with nanometer resolution, while fluorescence can facilitate the identification of their constituent parts. In addition, nanophotonic effects, such as fluorescence quenching or enhancement due to the AFM tip, can be used to increase the optical resolution beyond the diffraction limit. For the first time, this development grants access to the effects of precise physical impact on biomaterials like cells and its effects simultaneously measured by fluorescence parameters. The combination of the MicroTime 200 STED with an AFM has currently been realized for three different AFMs:
Spectrograph add-on
 Adding a spectrograph (SR-163 from Andor) along with a single molecule sensitive CCD camera to the MicroTime 200 STED opens up new prospects in the investigations of single molecules. The spectrograph is attached via a multimode fibre to an exit port of the main optical unit of the MicroTime 200 STED. Inside the main optical unit, a suitable optical element is used to direct a defined part of the emitted light towards the exit port. An easy exchange of the optical element (100% mirror, 50/50 beamsplitter, ...) allows for the customization of the experimental conditions. Depending on the context of the experiment, a defined part of the fluorescence is utilized to acquire the spectral information. Intensity fluctuations, fluorescence decay properties and spectral data of a single molecule can be obtained simultaneously. Both, the observation of spectral changes as a function of time, and the comparison of spectra from different molecules are possible with this combination.
Adding a spectrograph (SR-163 from Andor) along with a single molecule sensitive CCD camera to the MicroTime 200 STED opens up new prospects in the investigations of single molecules. The spectrograph is attached via a multimode fibre to an exit port of the main optical unit of the MicroTime 200 STED. Inside the main optical unit, a suitable optical element is used to direct a defined part of the emitted light towards the exit port. An easy exchange of the optical element (100% mirror, 50/50 beamsplitter, ...) allows for the customization of the experimental conditions. Depending on the context of the experiment, a defined part of the fluorescence is utilized to acquire the spectral information. Intensity fluctuations, fluorescence decay properties and spectral data of a single molecule can be obtained simultaneously. Both, the observation of spectral changes as a function of time, and the comparison of spectra from different molecules are possible with this combination.
Presentation of the MicroTime 200 STED
Interview with Stefan W. Hell
MicroTime 200 - demonstration of single molecule microscopy
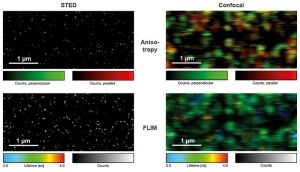
Single Molecule Imaging
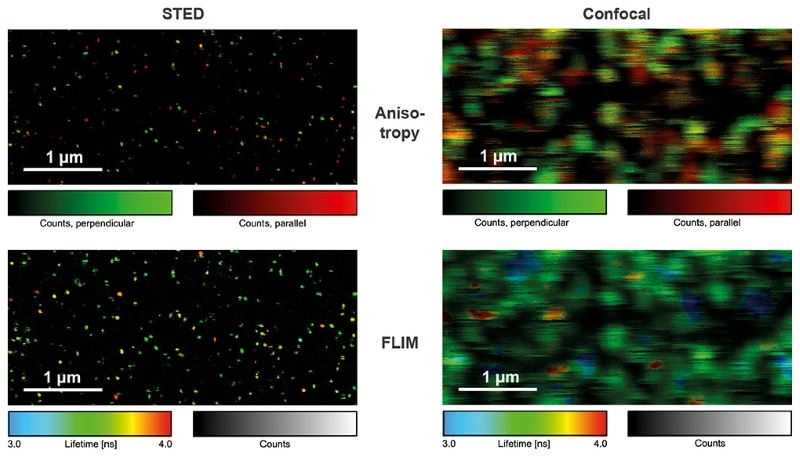
Detecting the emission of single molecules is important in biochemistry, drug development, and fundamental research. Single molecule sensitive systems aim at minimizing the number of optical elements to maximize light throughput, which is why piezo scanning systems were commonly used. With the FLIMbee add-on, the MicroTime 200 retains its outstanding single molecule sensitivity, but now with a much higher scanning speed. The images shown in this example were obtained from single ATTO 655 molecules bound to a glass coverslip, imaged under STED and confocal conditions with polarization- and time-resolved data acquisition. By using Pulsed Interleaved Excitation (PIE), STED and confocal data can be acquired quasi simultaneously, making the analysis of blinking and bleaching of single molecules straightforward.
Set-up:
- MicroTime 200
- Galvo scanner: FLIMbee
- TCSPC unit: TimeHarp 260 PICO
- Excitation: 640 nm with LDH-D-C-640
- Data analysis: SymPhoTime 64
FLIM-STED
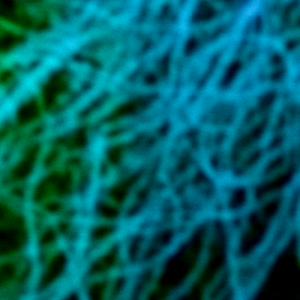
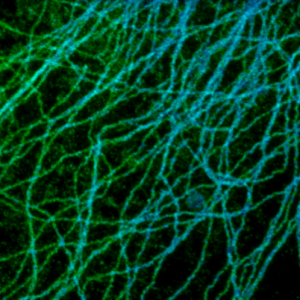
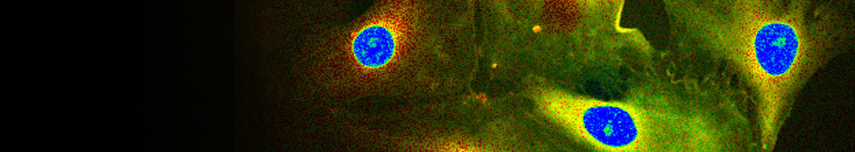
Super-resolution STED imaging is now a well established tool in molecular biology. Single molecules, cells, and tissue samples can be investigated using a wide variety of parameters, like emission color, polarisation, and fluorescence lifetime. With the MicroTime 200 STED, super-resolution FLIM images can be easily recorded while the high scan speed of the FLIMbee helps reducing phototoxicity and bleaching during STED imaging.
The example shows an image of the microtubule network in an adherent cell. The higher resolution of STED allows studying the interaction within the network in much more detail.
Set-up:
- MicroTime 200 STED
- Galvo scanner: FLIMbee
- TCSPC unit: TimeHarp 260 PICO
- Excitation: 640 nm with LDH-D-C-640
- STED laser: 765 nm with VisIR-765 STED
- Data analysis: SymPhoTime 64
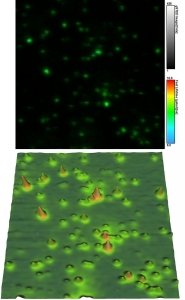
Simultaneous Measurement of Topological and Fluorescence Parameters
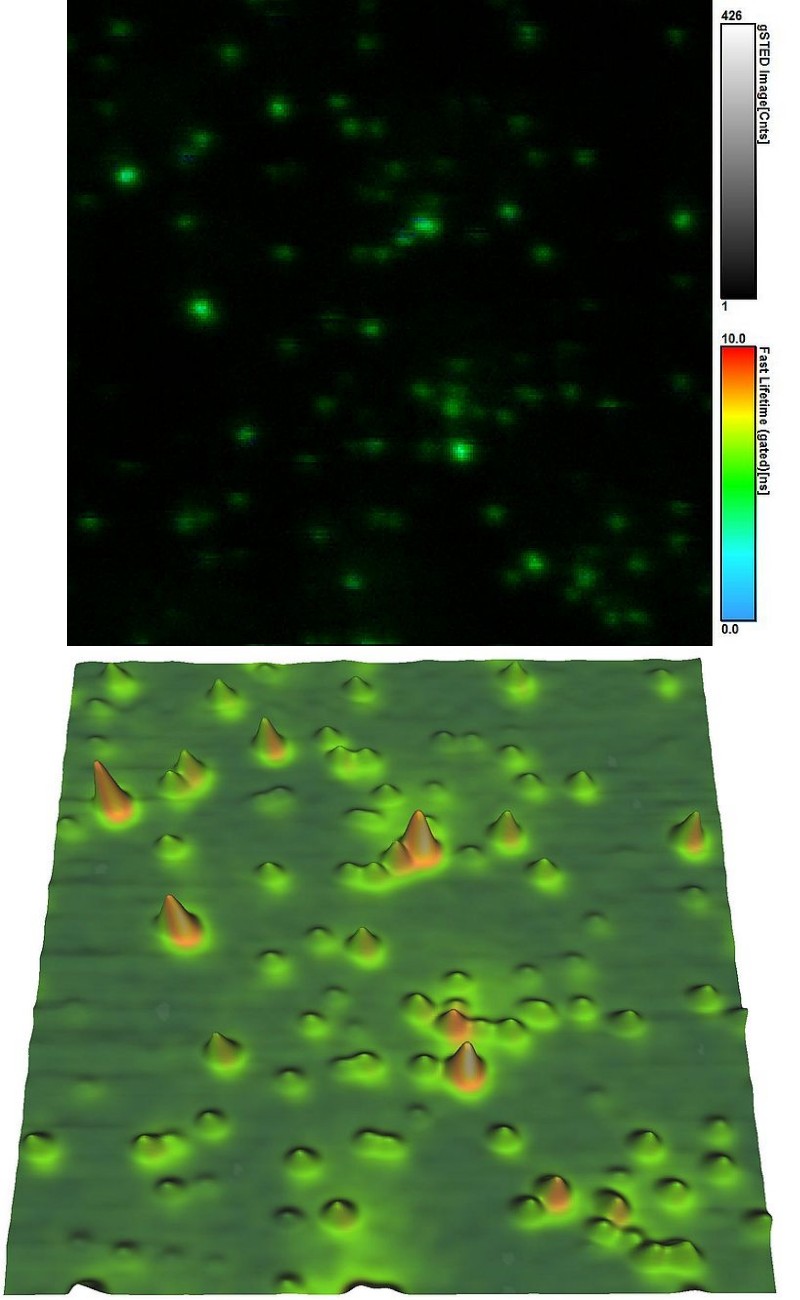 In this example, both fluorescence and topological information of dye-labeled beads were recorded simultaneously from the same sample area. Fluorescence parameters like emission intensity and lifetime shown in the top figure were obtained with STED super-resolution (<50 nm) on a MicroTime 200 STED. A JPK NanoWizard 3 atomic force microscope (AFM) was interfaced to the time-resolved fluorescence microscope, which allowed recording a height map from the same sample are (as shown in the lower picture).
In this example, both fluorescence and topological information of dye-labeled beads were recorded simultaneously from the same sample area. Fluorescence parameters like emission intensity and lifetime shown in the top figure were obtained with STED super-resolution (<50 nm) on a MicroTime 200 STED. A JPK NanoWizard 3 atomic force microscope (AFM) was interfaced to the time-resolved fluorescence microscope, which allowed recording a height map from the same sample are (as shown in the lower picture).
The easy-to-use combination of fluorescence lifetime imaging (FLIM) with topological information from AFM offers exciting avenues for investigating the structure-fluorescence interactions in e.g., biomaterials.
Set-up:
- Sample: 20 nm beads labeled with dye
- Excitation: 640 nm
- STED laser: 765 nm
- PDL 828 “Sepia II” with SOM 828-D oscillator module
- JPK NanoWizard 3 AFM
Multicolor Nanorulers for Super-resolution Microscopy

.png)
Nanorulers labeled with two different dyes are available to evaluate the resolution of diffraction-limit-breaking microscopes. This example shows GATTAquant STED 140RYR nanorulers, labeled with two red (ends) and a yellow dye (middle part). The distance between a red and yellow marker is 70 nm, while two red dyes on the same nanoruler are separated by a distance of 140 nm.
Set-up:
- Excitation: 595 and 640 nm
- Depletion: 765 nm
- PDL 828 "Sepia II" with SOM 828-D oscillator module
The samples were kindly provided by GATTAquant GmbH
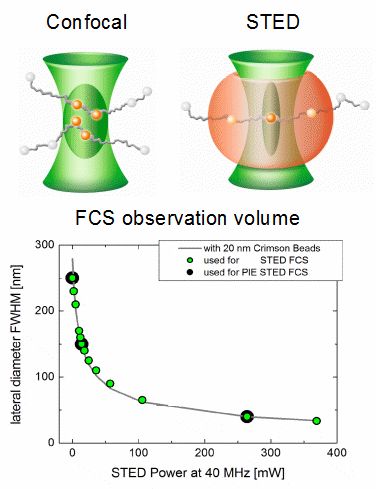
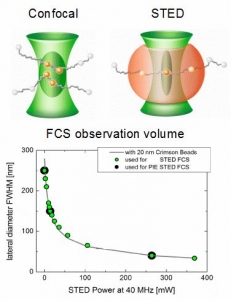
PIE-STED Fluorescence Correlation Spectroscopy
Fluorescence Correlation Spectroscopy (FCS) is a well established method for studying the diffusion behavior of molecules in solution or membranes. Under confocal conditions, the minimal size of the FCS observation volume is restricted by the diffraction limit. However, this limit can be circumvented by employing Stimulated Emission Depletion (STED) and the observation volume can be shrunk in a gradual manner by increasing the STED laser intensity.
At low STED laser powers, a small increase in intensity leads to a rapid shrinking of the lateral observation area diameter. Going from 0 to 50 mW reduces the spot diameter from 250 to less than 100 nm, depending on the fluorophore used. At high STED powers, however, increases in intensity will result in slower shrinking until a diameter below 50 nm can be reached.
Shrinking the detection volume helps in overcoming averaging issues in long transit paths and also enables determining the type of hindered diffusion behavior of fluorophores in lipid membranes. Furthermore, using a fully Pulsed Interleaved Excitation (PIE) illumination scheme for excitation and STED lasers allows collecting FCS data under both confocal and STED conditions quasi-simultaneously. PIE-STED-FCS allows for a straightforward check whether the STED laser has an influence on the investigated diffusion dynamics.
Set-up:
- Excitation: 640 nm
- Depletion: 765 nm
- PDL 828 "Sepia II" with SOM 828-D oscillator module
Reference: Koenig, M. et al., Proceedings of SPIE, Vol.9712, 97120T (2016)
Cytoskeleton – microtubules
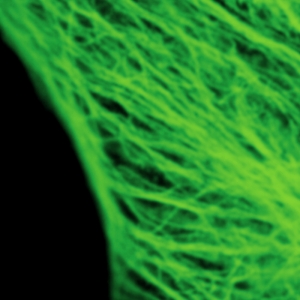
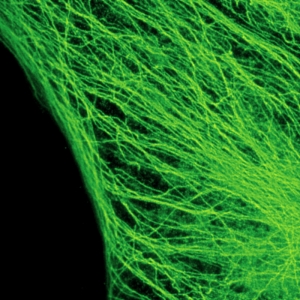
The example shows tubulin structures in a HeLa cell labeled with Abberior STAR635p. Super-resolution permits the clearer distinction of the filamentous structures in the STED image.
Set-up:
- Excitation: 640 nm
- Depletion: 765 nm
FLIM-STED pattern matching for dual species separation
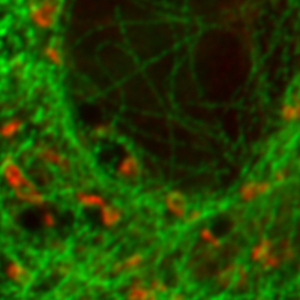
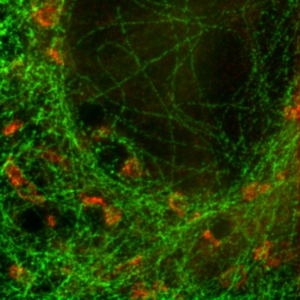
Two fluorescent species can be distinguished by pulsed interleaved excititation (PIE) with two different diode lasers at 640 and 660 nm. Fluorescence is detected in two spectral bands. Fluorescence pattern matching was used to identify the contributions of each fluorophore. The example shows part of a U2OS cell labeled for tubulin with Abberior STAR 635p (green) and giantin with Atto647N (red) utilizing a single STED laser wavelength.
Set-up:
- Excitation 640 and 660 nm
- Depletion: 765 nm
- PDL 828 "Sepia II" with SOM 828-D oscillator module
Sample courtesy of Markus Sauer, University of Würzburg, Germany
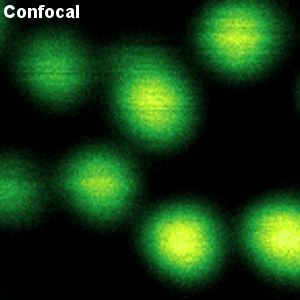
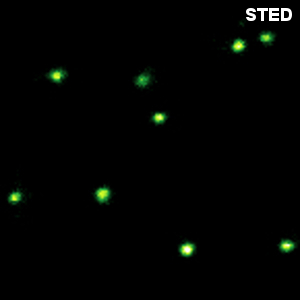
Crimson beads
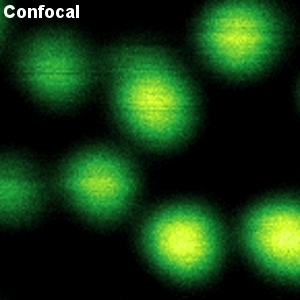
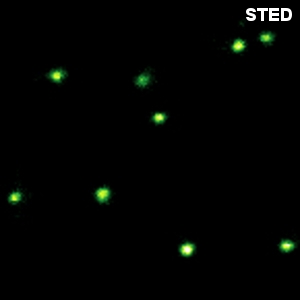
The example shows a comparison between confocal and STED images of Crimson beads (20 nm diameter). Fluorescent beads are often used to evaluate the resolution of a microscope. The FWHM in the confocal image is 260 nm, and in the STED image it is 38 nm. The resolution enhancement is clearly visible.
Set-up:
- Excitation: 640 nm
- Depletion: 765 nm
Evaluating resolution with DNA origami


Specifically labeled DNA origami is now commonly used to evaluate the resolution of diffraction-limit-breaking microscopes. Here, origami with two rows of 11 dye molecules with a distance of 71 nm was used. Image size: 4 µm x 4µm
Set-up:
- Excitation: 640 nm
- Depletion: 765 nm
The samples were provided by GATTAquant GmbH and the Tinnefeld lab, TU Braunschweig, Germany
The MicroTime 200 STD is a time-resolved confocal microscope with single molecule sensitivity and super-resolution capabilities. Hence, numrous applications are possble with this instrument, including:
- Stimulated Emission Depletion Microscopy (STED) / gated STED
- Single Molecule Spectroscopy / Detection
- Time-Resolved Fluorescence
- Fluorescence Lifetime Imaging (FLIM)
- Phosphorescence Lifetime Imaging (PLIM)
- Fluorescence Correlation Spectroscopy (FCS)
- Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Foerster Resonance Energy Transfer (FRET)
- Dual-focus Fluorescence Correlation Spectroscopy (2fFCS)
- Scanning FCS (sFCS)
- Pulsed Interleaved Excitation (PIE)
- Fluorescence Anisotropy (Polarization)
- Pattern Matching Analysis
- Time-Resolved Photoluminescence (TRPL)
- TRPL Imaging
- Antibunching
The following documents are available for download:
- Brochure about the MicroTime 200 STED
- Brochure about the MicroTime 200
- Brochure about the FLIMbee galvo scanner
Related technical and application notes:
- Technical note: Spectrograph Add-on for the MicroTime 200
- Technical note: Combination of MicroTime 200 with Asylum AFM
- Technical note: Combination of MicroTime 200 with Bruker AFM
- Technical note: Combination of MicroTime 200 with JPK AFM
- Technical note: Sample temperature controller for the MicroTime 200
- Technical note: Time-Correlated Single Photon Counting (TCSPC)
- Technical note: FLIM. Scanning Speed vs. Excitation Rate
- Technical note: Phosphorescence Lifetime Imaging Microscopy (PLIM) Measurements. Practical Aspects
- Application note: Quantitative Fluorescence Correlation Spectroscopy
- Application note: Time-gated Fluorescence Correlation Spectoscopy
- Application note: Absolute diffusion coefficients
- Application note: Two-Focus Fluorescence Correlation Spectroscopy (2fFCS)
- Application note: Fluorescence Lifetime Correlation Spectroscopy (FLCS)
- Application note: Fluorescence Lifetime Imaging (FLIM) in Confocal Microscopy
- Application note: Imaging of molecular distances using FLIM-FRET
- Application note: FRET analysis with Pulsed-Interleaved Excitation (PIE)
- Application note: Two-photon excitation using the MicroTime 200
Latest 10 publications referencing MicroTime 200, SymPhoTime
The following list is an extract of 10 recent publications from our bibliography that either bear reference or are releated to this product in some way. Do you miss your publication? If yes, we will be happy to include it in our bibliography. Please send an e-mail to info@picoquant.com containing the appropriate citation. Thank you very much in advance for your kind co-operation.




 Get in touch
Get in touch

__small.png)